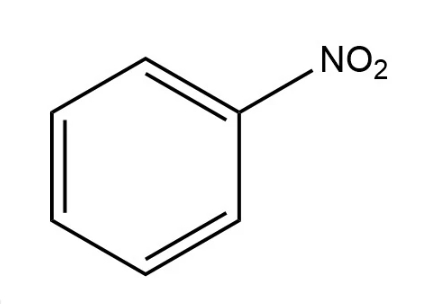
Nitrobenzene
Chinese name nitrobenzene
Foreign name Nitrobenzene
Chemical formula C6H5NO2
Molecular Weight: 123.109CAS
Registration number: 98-95-3EINECS
Registration number 202-716-0
Boiling point 210 to 211 °C
Water solubility, difficult to dissolve in water
Density 1.205 g/cm³
Flash point 88 °c
Security Description S7; S16; S27; S28; S36/37;S45; S61 Hazard symbol T; N
Chinese title nitrobenzene
English title nitrobenzene
English aliases more
Nitrobenzene physicochemical properties
Density 1.205
boiling factor 210-211 °C(lit.)
melting factor 5-6 °C(lit.)
Molecular Formula C6H5NO2
Molecular Weight: 123.10900
Flash factor one hundred ninety °F
Exact mass 123.03200
PSA 45.82000
LogP 2.11800
Appearance qualities yellow liquid
Vapor density 4.2 (vs air)
Vapour stress 0.15 mm Hg ( 20 °C)
refractive index n20/D 1.551(lit.)
Storage conditions
Storage precautions Store in a cool, ventilated warehouse. Keep away from tinder, warmness sources. Keep the container tightly sealed. It ought to be saved one by one from oxidants, decreasing agents, alkalis, and fit for human consumption chemicals, and combined storage need to be avoided. Equipped with furnace battle gear of corresponding sorts and quantities. Storage areas have to be outfitted with emergency therapy tools for leakage and appropriate containment materials.
stability
1. Chemical properties: fantastically steady to acids and alkalis. It can volatilize with water vapor and has vulnerable oxidation. Iron, zinc and different metals react with hydrochloric acid, or use nickel, copper, silver, etc. as catalysts, stress for reduction, to generate aniline. React with the blended acid of sulfuric acid and nitric acid to generate dinitrobenzene or trinitrobenzene. Chlorination is carried out in the presence of iodine or magnesium chloride to shape m-clonitrobenzene. The chlorination response is carried out in the presence of ferric chloride, and 2,5-dichloronitrobenzene is fashioned at room temperature. At a hundred °C, 2,3,5,6-tetrachloronitrobenzene is generated. HCB is generated above one hundred °C. The interplay with oleum sulfuric acid ordinarily produces m-nitrobenzenesulfonic acid. It reacts with potassium hydroxide to shape o-nitrophenol and a small quantity of p-nitrophenol. Nitrobenzene can react with Grignard's reagent.
2. Stability Stable
3. Forbidden elements robust oxidants, ammonia, amines, etc
4. Aggregation risks do no longer polymerize
5. Decomposition merchandise nitrogen oxides


Related News
Submitted successfully
We will contact you as soon as possible