CAS 288-32-4
Melting point 88-91 °C (lit.) Boiling point 256 °C (lit.) Density 1.01g/mLat20 °CVapor pressure< 1mmHg (20 °C) Refractive index 1.4801Flash point 293 °F Storage conditions StChemicalbookorebelow+30 °C. Solubility H2O: 0.1Mat20 °C, clear, colorless morphology, crystalline acidity coefficient (pKa) 6.953 (at25 °C)
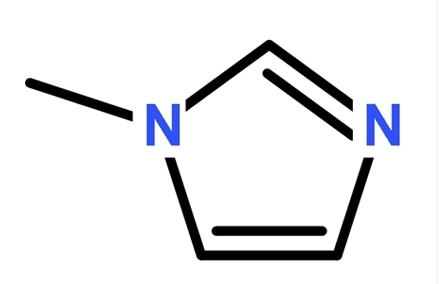
Imidazole, molecular formulation C3H4N2, is an natural compound, a kind of diazole, a five-membered fragrant heterocyclic compound containing two meta-nitrogen atoms in its molecular structure. The unshared electron pair of the 1-position nitrogen atom in the imidazole ring participates in the cyclic conjugation, and the electron density of the nitrogen atom decreases, making the hydrogen on this nitrogen atom handy to depart as hydrogen ions.
Imidazole is acidic, additionally alkaline, can shape salts with sturdy bases, the chemical residences of imidazole can be summarized with the synthesis of pyridine and pyrrole, these two structural devices exactly in the enzyme histidine as an acyl switch reagent in the catalysis of lipid hydrolysis performs an essential role. Derivatives of imidazole exist in the organic organism and are greater essential than imidazole itself in scientific lookup and industrial production, such as DNA, hemoglobin, etc.
The electron cloud density on imidazole N-3 is large, so alkylation typically happens first on this nitrogen atom. The product of a single alkylation can produce a nitrogen atom comparable to that in pyridine via tautomerization, so it can be in addition reacted to produce the product of dialkylation imidazolium salt.
The acylation response of imidazole normally happens on N-3, however due to the fact the acyl crew is an electron-absorbing group, the response can be managed in the monoacylation stage, and the product is N-acylimidazole.
The energetic hydrogen of imidazole can decompose Grignard's reagent to shape the N-magnesium salt of imidazole, which is isomerized to reap C-2-substituted imidazole. The latter is dealt with with methyl iodide to produce 1,2-dimethylimidazole.
Imidazole can be brought to the diolenophile, forming a 3-amium amphoteric ion, and then including with any other molecule of the diene nucleophile to shape a product of C-2 cyclization. For example, 1-methyl-2-ethylimidazole reacts with two molecules of dimethyl butynedioate to acquire 4methyl 8α-ethyl-1-methyl-1,8α-dihydroimidazol[1,2-a]pyridine-5,6,7,8-tetracarboxylate.


Related Products
Submitted successfully
We will contact you as soon as possible
Related News
Submitted successfully
We will contact you as soon as possible