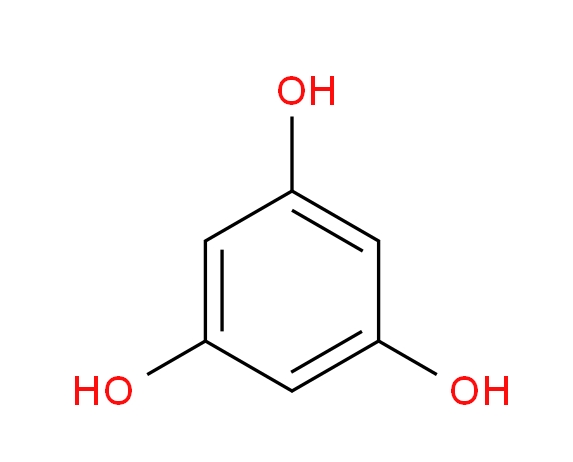
Phloroglucinol
1. Properties: white or light yellow crystal powder.
2. Melting point(°C):218
3. Boiling point (°C): sublimation
4. Relative density (water = 1): 1.46
5. Relative vapor density (air = 1): 4.3
6. Heat of combustion (kJ/mol):-2657.2
7. Octanol/water partition coefficient: -0.19
8. Solubility: slightly soluble in water, soluble in ethanol, ether, benzene.
Chemical properties
Formaldehyde and resorcinol react under alkaline conditions to form orange-red compounds, which are colorimetric at a maximum absorption wavelength of 460nm to detect low traces of formaldehyde in textile and clothing.
Since resorcinol can undergo enol and ketone form tautomerism, it can react with ammonia as follows:
Ketone reaction of resorcinol
Under the action of ammonia, m-phenyltriamine is obtained by reaction in ketone, and the amine is hydrolyzed in the aqueous solution of acid to obtain resorcinol. Resorcinol can also be reacted in the form of enols:
Ecological data
Ecotoxicity
LC50: 630mg/L (48h) (fish)
EC50: 0.6 mg/L (48h) (Daphnia)
IC50: 200mg/L (72h) (algae)
Molecular structure data
Molar refractive index: 31.89
Molar volume (cm3/mol): 84.7
Isotonic specific volume (90.2K): 252.3
Surface tension (dyne/cm): 78.6
Polarizability (10-24cm3): 12.64
Toxicological data
1. Acute toxicity: rat oral LD50: 5200mg/kg; rat transperitoneal LD50: 3180mg/kg; Rat percutaneous LD50: 4850mg/kg; Mouse oral LD50: 4550mg/kg; Mice transperitoneal LD50: 4050mg/kg; Mouse percutaneous LD50: 991mg/kg; Dog transvenous LDLo: >250mg/kg; [1]
2. Reproductive toxicity: rat percutaneous TDLo: 5mg/kg (1 day after conception of the mother mouse); [1]
3. Mutagenicity: yeast cell gene conversion and mitosis recombination test: 3mg/L; Cytogenetic analysis of hamster ovaries: 3 mg/L;


Related News
Submitted successfully
We will contact you as soon as possible