CAS 80756-85-0
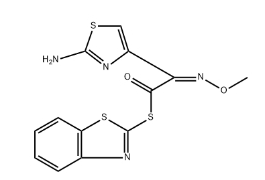
S-benzothiazol-2-yl (Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)thioacetate
S-2-Benzothiazoyl-2-amino-alpha-methoxyimino-4-thiazoleacetate(MAEM)
S-2-benzothiazoyl-2-amino-α-methoxyimino-4-thiazoleacetate
(Benzothiazol-2-yl)-(Z)-2-methoxyimino-2-(2-amino-thiozol-4-yl) thioacetate
S-2-Benzothiazolyl-2-amino-alpha-(methoxyimino)-4-thiazolethioacetate
2-Mercaptobenzothiazolyl (2-Aminothiazolyl)-Methoxyimino Acetate
(BENZOTHIAZOL-2-YL)-2-(2-AMINOTHIZAZOL-4-YL)-(Z)-2-METHOXYIMINO THIOACETATE
2-Amino-α-(methoxyimino)-4-thiazoleethanethioic acid, S-2-benzothiazolyl ester
S-(Benzothiazol-2-yl) (Z)-(2-aminothiazol-4-yl) (methoxyimino) thioacetate
S-(Benzothiazol-2-yl) 2-amino-α-(methoxyimino) thiazole-4-thioacetate
Appearance: Light yellow crystalline powder
Melting Point: 128-130 °C(lit.)
Boiling Point:563.2±42.0 °C(Predicted)
Density 1.63
refractive index1.6200 (estimate)
Storage conditions: Refrigerator, Under Inert Atmosphere
Solubility soluble in chloroform (a little), methanol (a little)
pKa1.22±0.10(Predicted)
Morphological solids
The color is pale yellow
Stability: Moisture sensitive
InChIKeyCOFDRZLHVALCDU-YVLHZVERSA-N
CAS DataBase Reference80756-85-0
Brief introduction
AE-active ester, chemical name 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-thioacetic acid benzothiazole ester. Appearance: white or light yellow crystalline powder, melting point 128130 °C. Low toxicity and bitter taste, soluble in acetone, tetrahydrofuran, slightly soluble in acetonitrile, dichloromethane, insoluble in water, and can be burned in case of open flame.
apply
AE-active ester is one of the important side chains of semi-synthetic cephalosporin antibiotic drugs, and is an indispensable intermediate for the synthesis of third- and fourth-generation semi-synthetic cephalosporins such as cefotaxime sodium, ceftriaxone sodium, cefpime, cefpiro, etc.
apply
AE-active ester is an ester derivative, which can be used as the main raw material for the production of cefttriazine, cefotaxime sodium and other drugs.
preparation
In a 500ml four-mouth flask equipped with mechanical stirring, a constant pressure dropping funnel, a reflux condenser tube and a thermometer, add 20g (0.0995mol) of ammothiazome acetic acid, add dibenzothiazolethiazole thioether, pyridine and 100ml of dichloromethane in proportion, start stirring, slowly add 80ml of triphenylphos dichloromethane solution dropwise under room temperature and vigorous stirring, add it, stir the reaction at a temperature of 20~25 °C for 3h (t1), cool in an ice bath, filter, wash the filter cake with methanol, vacuum drying, 29.1g (0.08314mol, theoretical value of 0.0995mol) of AE-active ester was obtained, the yield was 83.6%, and the content was 98.6% (LC); Under vigorous stirring of the filtrate, slowly add 50ml of dichloromethane solution of bis(trichloromethyl) carbonate dropwise, add it, continue the reaction at 20~25 °C for 3 hours (t2), after the reaction, filter, filter cake washed with methanol, and obtain 16.6g of dibenzothiazole thioether (0.05mol, the theoretical value is the sum of the amount recovered and the amount not participating in the reaction, here it should be 0.0995×1.2-0.0995+0.0995/2=0.07mol), The yield was 71.43%, the content was 98.1% (LC), the filtrate was concentrated, and the methanol was recrystallized to obtain 22.9g (0.0874mol, theoretical value of 0.1194mol), the yield was about 73.2%, and the content was 98.3% (LC).

Related News
Submitted successfully
We will contact you as soon as possible