CAS 71-36-3
Chinese Name:
Butanol
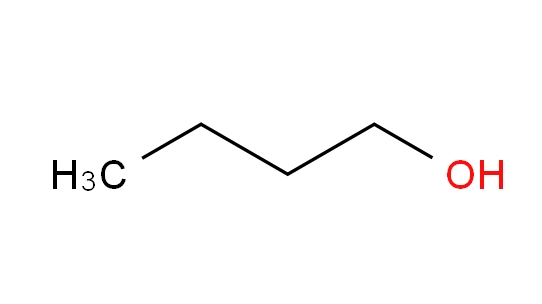
Name:1-Butanol CAS No.:71-36-3 Molecular Formula:
C4H10O isomer
Molecular weight:
74.1216
EINECS number:
200-751-6
Category:
Alcohols, other pharmaceutical intermediates, cosmetic raw materials
Character description: colorless obvious liquid. Melting factor -90.2C, boiling factor 117.7 °C, relative density 0.8098 (20/4C), refractive index 1.3993, flash factor 35.35.5 °C, spontaneous ignition factor 365 °C. The solubility in water at 20 °C is 7.7% (mass), and the solubility of water in n-butanol is 20.1% (mass). Miscible with ethanol, ethyl and different natural solvents. Vapor varieties an explosive combination with air, with an explosion restrict of 1.45-11.25 (volume). It can shape an azeotropic combination with water, its boiling factor is ninety two °C, and the water content material is 37%. It has a one of a kind aroma like wine.
Production method: There are three important industrial manufacturing techniques of n-butanol: fermentation method, propylene carbon-based synthesis technique and acetaldehyde condensation method
1. Fermentation method: grain, cereals, dried potato or molasses and different uncooked materials are crushed, delivered water to make fermentation broth, cooled earlier than and after high-pressure steam treatment, introduced pure acetone-butanol strains, and fermented at 36-37 °C. During fermentation, gases containing carbon dioxide and hydrogen are produced. The fermentation broth consists of ethanol, butanol, acetone, generally in a ratio of 6:3:1. After distillation, butanol, acetone and ethanol can be got respectively, and can additionally be used without delay as a complete solvent barring separation.
2. Radical synthesis method, specifically the Reppe process, coke fuel to achieve carbon monoxide and fuel gas, and propylene underneath excessive stress and the presence of drill or cash catalyst for carbon-based synthesis of n-butyraldehyde, hydrogenation and fractionation to reap n-butanol.
3. Alkaldehyde condensation is derived from two molecules, which are condensed and dehydrated to achieve crotonaldehyde, which is hydrogenated at one hundred eighty °C and 0.29MPa to produce n-butanol in the presence of nickel-chromium catalysis. The spinoff n-butanol can be used to produce isooctanol.
Alkaldehyde condensation legally produces 1tJ alcohol consumption 98.5%, acetaldehyde 1300kg, hydrogen 674kg.
Uses, basically used in the manufacture of phthalic acid, aliphatic diacids and phosphoric acid n-butyl cool plasticizers, they are extensively used in a variety of plastic and rubber products. It is additionally a uncooked cloth for the training of butyraldehyde, butyric acid, butylamine and butanone lactate in natural synthesis. It is additionally an extractant for oils and fats, tablets (such as antibiotics, hormones and vitamins) and fragrances, and an additive for alkyd resin coatings. It can additionally be used as a solvent for natural dyes and printing inks, and a dewaxing agent.


Related News
Submitted successfully
We will contact you as soon as possible