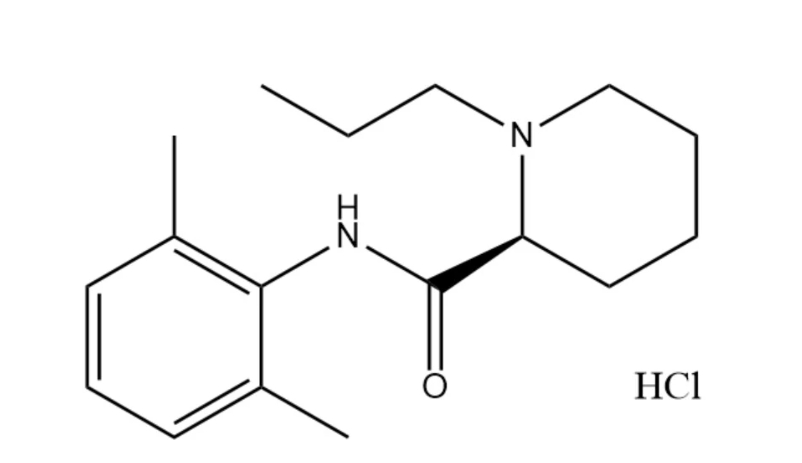
Pharmacological action
Since the discovery that long-acting local anesthetics can induce cardiac arrest, people have been looking for safer alternatives with less fat solubility. Ropivacaine is such a new long-acting amide local anesthetic, which has a long duration of action and has anesthetic and analgesic effects. Its pharmacological characteristics are low cardiotoxicity, sensory block and motor block are clearly separated, and it has peripheral vasoconstriction effect. Therefore, the drug is particularly suitable for postoperative analgesia and obstetric anesthesia.
Indications
Surgical anesthesia: epidural, including caesarean section (acute pain control), continuous epidural infusion, or intermittent single doses, such as postoperative or labor pain (regional block).
contraindication
Patients allergic to amide local anesthetics are contraindicated with ropivacaine injection.
Notes
The administration of regional anesthesia must be carried out on the basis of well-staffed and well-equipped personnel. Medications and equipment for monitoring and emergency resuscitation should be readily available. Intravenous access should be established before major anesthesia is administered. Clinical staff should be appropriately trained and familiar with the diagnosis and treatment of side effects, systemic toxicity, and other complications.
Some local anesthesia, such as head and neck injections, have a higher incidence of serious adverse effects, independent of the local anesthetic used. Special care should be taken in patients who are elderly or have other serious medical conditions that require regional anesthesia. In order to reduce the potential risk of serious adverse effects, every effort should be made to improve the patient's condition before anesthesia is administered, and the dose of the drug should be adjusted accordingly.
Since ropivacaine is metabolized in the liver, it should be used with caution in patients with severe liver disease, and the dose should be reduced when repeated use due to delayed excretion of the drug. Typically, patients with renal insufficiency do not need to adjust the dose if they are treated with a single dose or for a short period of time. Patients with chronic renal insufficiency are associated with acidosis and hypoproteinemia, and the possibility of systemic toxicity is increased.
Epidural anesthesia can produce hypotension and bradycardia, such as pre-infusion expansion or vasopressor drugs, can reduce this side effect, once hypotension can be treated with 5-10 mg of ephedrine intravenous, if necessary, repeat the drug.
Drug interactions
Patients receiving other local anesthetics or drugs associated with amide structures should be cautious when using ropivacaine injections at the same time, as toxic effects can be cumulative.
Pregnancy and breastfeeding
pregnancy
The effect of the drug on reproduction has been tested in rabbits and rats. The two generations of rats tested did not show that fertility and general reproductive behavior were affected by the drug. After the administration of the highest dose of ropivacaine, the number of deaths of pups within three days of giving birth increased due to its toxic effect on pregnant mothers, ranking as the second leading cause of death in newborn litters.
Teratogenic tests in rats and rabbits did not show any adverse effects of ropivacaine on organogenesis and early fetal development, and studies of perinatal and postpartum rats at the maximum tolerable dose did not show any effect on late fetal development, delivery, lactation, neonatal viability and offspring growth.
Another perinatal and postnatal study in rats compared ropivacaine with bupivacaine and found that toxic effects on pregnant mothers were observed with bupivacaine at much lower doses and low free plasma concentrations.
There are no clinical trials on the effects of prenatal maternal use of ropivacaine on fetal growth, so ropivacaine should only be used in pregnant women if the benefits to the fetus outweigh the harms.
The use of ropivacaine in delivery as an obstetric anesthesia or analgesia has been well documented without any side effects.
suckle
The secretion of ropivacaine or its metabolites in human milk has not been studied. Based on the ratio of milk/plasma concentration in rat experiments, the daily intake of young mice is estimated to be 4% of the dose of their mother rats. Assuming that the human milk/plasma concentration ratio is the same as in rats, the amount of ropivacaine given to breastfed infants is much lower than the dose received in the pregnant woman's uterus at the time of delivery.
Impact on driving and operating machinery
Even in the absence of significant central nervous system toxicity, local anesthesia can slightly affect mental condition and ataxia coordination, and can temporarily impair movement and flexibility, and these effects are dose-dependent.


Related News
Submitted successfully
We will contact you as soon as possible