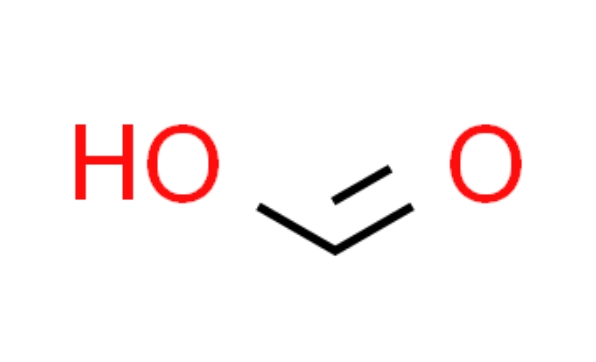
Formic acid is an organic substance with the chemical formula HCOOH and a molecular weight of 46.03 , commonly known as formic acid, which is the simplest carboxylic acid. A colorless liquid with a pungent odor. Formic acid is a weak electrolyte, but its aqueous solution is weakly acidic and corrosive, which can stimulate skin blistering. It is usually found in the secretions of bees, certain ants, and caterpillars. It is an organic chemical raw material, also used as a disinfectant and preservative.
Formic acid was first developed by J. J. -L. Guy-Lussac is prepared by decomposition of oxalic acid. In 1855~1856, M. Berthelaw directly prepared sodium formate with sodium hydroxide and carbon monoxide, and T. Gold-Schmidt was the first to prepare formic acid from sodium formate by hydrolysis. This method began to be used in industrial production in Europe in 1896 and is still used in small batch production. In 1980, Scientific Design Corporation, Bethlehem Steel Company and Leonard Company successfully developed a method for the carbonylation of methanol to produce formic acid, and a plant with an annual output of 20 thousand tons of formic acid was put into operation. In addition, formic acid can also be recovered from the by-product of oxidation of acetic acid from light oil.
In hydrocarbon and gaseous state, formic acid occurs in the form of dimers bound by hydrogen bonding. In the gaseous state, hydrogen bonding results in a large deviation between the formic acid gas and the ideal gas equation of state. Liquid and solid formic acid consists of a continuous stream of formic acid molecules bound by hydrogen bonding. Due to the special structure of formic acid, one of its hydrogen atoms is directly connected to the carboxyl group. It can also be seen as a hydroxyformaldehyde.


Related Products
Related News
Submitted successfully
We will contact you as soon as possible