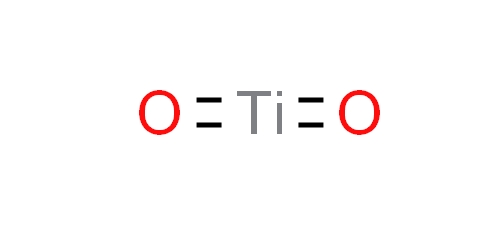
CAS 1317-80-2
Experimental characteristics
LogP -0.23760
PSA 0.00000
Merck 14,9472
Water soluble in hot concentrated sulfuric acid, in hydrofluoric acid and alkali. Insoluble in water.
boiling point 2900 °c
melting point 1840 °c
flash point 2500-3000°C
Color and properties of white amorphous powder, turn brown at high temperature. Aggregates are granular or dense in mass. dark red, brownish-red, yellow or orange-yellow, iron-rich black; The streaks are yellow to light brown. Diamond luster, iron rutile has a semi-metallic luster. Brittle
Titanium dioxide, is an inorganic compound, chemical formula TiO2, is a white solid or powder amphoteric oxide, molecular weight 79.866, non-toxic, the best opacity, the best whiteness and brightness, is considered to be the best performance white pigment in the world today. Titanium white has strong adhesion, is not easy to chemically change, and is always snow-white. Widely used in coatings, plastics, papermaking, printing inks, chemical fiber, rubber, cosmetics and other industries. It has a high melting point and is also used to make refractory glass, glazes, enamel, terracotta, high-temperature resistant experimental utensils, etc. Titanium dioxide can be extracted from rutile by acid decomposition or decomposed by titanium tetrachloride. Titanium dioxide has three allotropes in nature: rutile, anatase and plate titanium, in addition to several synthetic crystal forms.
Due to the high dielectric constant of titanium dioxide, it has excellent electrical properties. When determining certain physical properties of titanium dioxide, the crystallization direction of titanium dioxide crystals should be considered. For example, the dielectric constant of the rutile type, which varies with the direction of the crystal, is measured as 180 when parallel to the C axis, 90 at right angles to this axis, and its powder average is 114. The dielectric constant of anatase titanium dioxide is relatively low, only 48.
electrical conductivity
Titanium dioxide has the properties of semiconductors, its conductivity increases rapidly with temperature, and it is also very sensitive to hypoxia. Rutile titanium dioxide, for example, is still an electrical insulator at 20°C, but its conductivity increases by 107 times when heated to 420°C. Slightly reducing the oxygen content will have a special effect on its conductivity, according to the chemical composition of titanium dioxide (TiO2) conductivity < 10-10s/cm, while TiO1.9995 conductivity is as high as 10-1s/cm. Rutile titanium dioxide's dielectric constant and semiconducting properties are important to the electronics industry, which uses these properties to produce electronic components such as ceramic capacitors.


Related News
Submitted successfully
We will contact you as soon as possible